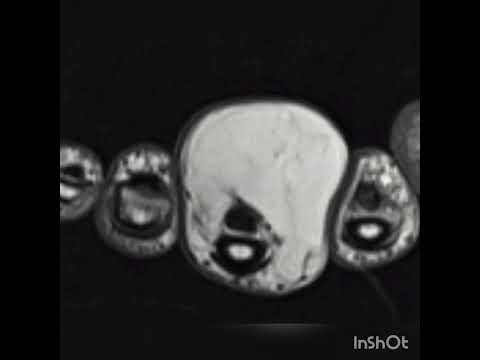
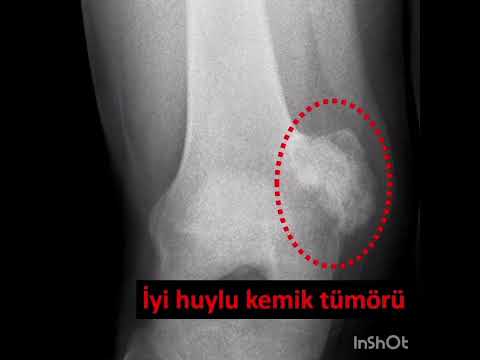
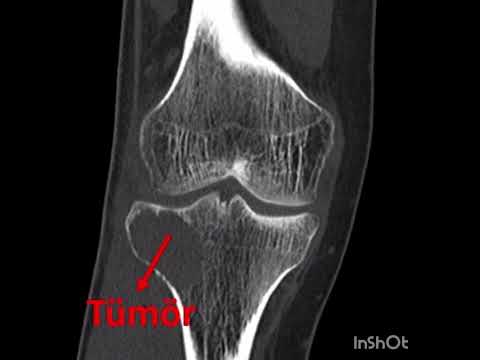
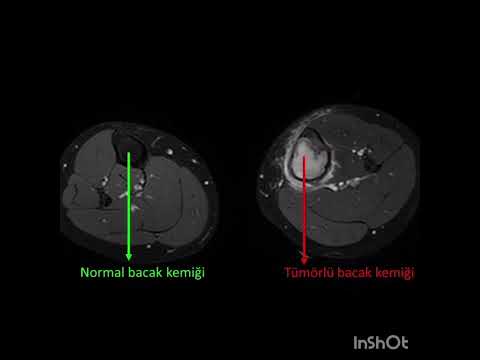
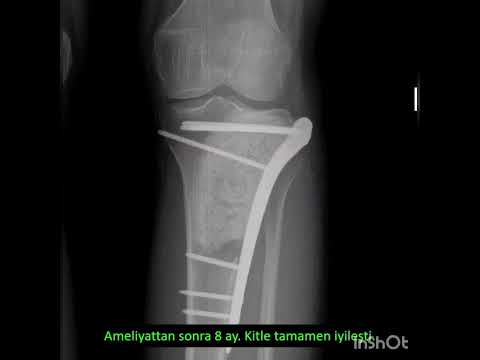
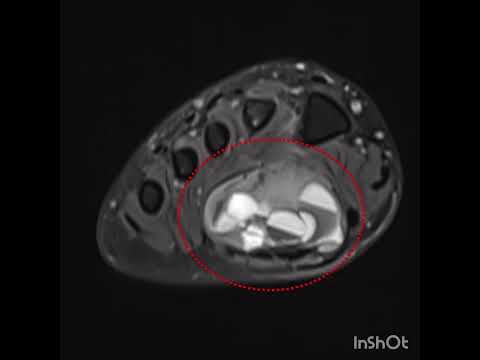
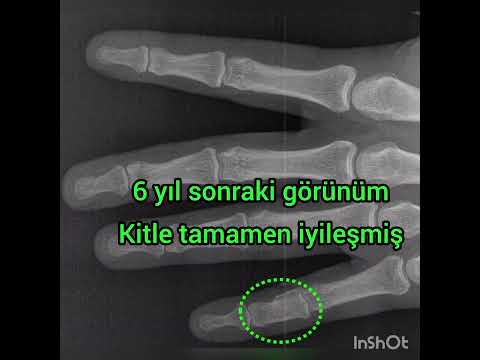
Can the arterial clamp method be used safely where a tourniquet cannot be used?
Ozgur Erdogan, Volkan Gürkan, Cavide Sönmez,
Tunay Erden, Sezen Atasoy, Fatih Yildiz, Bekir İnan, Adile Adilli
Blood
loss can occur during any surgical procedure. However, certain types of surgery
are associated with higher amounts of blood loss, which require transfusion.
Some of these procedures are kidney, hepatic, orthopedic and vascular
operations.1 Orthopaedic surgery, especially malignant tumour surgeries of the
extremities, results in severe blood loss, which causes difficulty in
dissection of the tumour and neurovascular structures, and consequently
prolonged surgical times, excessive blood transfusion and transfusion-related
complications. Tourniquet use solves this problem, however its use becomes
impossible if the surgical field is very proximal, where arterial clamp use
could be effective. It should be noted that tourniquet use can be dangerous and
in some instances may be contraindicated.2 Also, there is controversy about the
appropriate application time and pressure. Moreover, severe neurological and
muscular damage related to the use of a tourniquet has been reported.3 In
radical surgical procedures, arterial clamp application through an additional
small incision and vascular dissection, which can quickly close and open the
blood supply, may solve these problems. However, orthopaedic surgeons are often
unfamiliar with arterial clamp use or they are unsure about its reliability
and/or safety. The literature on arterial clamp use in malignant tumour
surgeries of the proximal parts of the extremities is lacking. This study aimed
to investigate the effectiveness and safety of arterial clamps in terms of
possible damage to the arterial wall. The main question motivating this work
was why we do not use the clamp method in orthopedic, oncological procedures in
patients where tourniquet application is impossible. The hypothesis was that
tourniquet use has less adverse effects on the arterial wall than the direct
application of an arterial clamp.
Methods
Methods
This animal study proposal was approved by the local ethics committee and local
animal experimental ethics committee (HADYEK) of the Bezmialem University, with
permit number: 2017/21. According to the guide, Institutional Animal Care and
Use Committee (IACUC)4 for the care and use of laboratory animals, principles
and animal rights were protected in this study. A local ethics committee,
approved by Bezmialem University Faculty of Medicine Ethics Committe, approved
this study: number 2016/154. In the study design, Guide for the Care and Use of
Laboratory Animals5 was used, and two attendant veterinarians controlled all
procedures. The investigators found it helpful to consult with experts
regarding statistical analysis for required animal numbers, and database
searches to identify potential alternatives to painful or distressing
procedures.6 Retrobulbar injection of no more than 200 μl of injectable anaesthetic solution (ketamine:xylazine) was
used, resulting in death within five seconds of cessation of injection,7 as
mentioned among the forms of euthanasia in the AVMA Guidelines for the
Euthanasia of Animals, 2020 edition. Twenty-one New Zealand male white rabbits
(eight months old, mean weight 3 kg, range 2.6–3.4 kg) were obtained from a
private farm, by veterinary faculty authority. The 21 rabbits were divided into
three groups: group I received a proximal femoral artery clamp; group II
received a proximal thigh tourniquet, and group III was the control group. The
rabbits were prepared in the supine position after
anaesthesia
using 2 mg/kg of intramuscular diazepam and 40 mg/kg ketamine, and they were
draped after shaving and cleaning their skin with betadine. In order not to
affect tumour necrosis factor alpha (TNF-α) values, only one
limb side was used in all subjects. In the clamp group, a proximal incision was
made anteromedially over the femoral neurovascular margin, and the skin,
subcutaneous tissue and deep fascia were incised. After dissecting the muscles
and exposing the neurovascular bundle, the femoral artery was dissected and
clamped with a microvascular clamp (Biemer vessel clip, 7-mm jaw length,
closing force 30 G, MCI-47-104, Medical Care Instruments, Manchester, UK). The
area where the clamp was applied was referred to as the ‘middle’. The location
1 cm proximally was denoted ‘proximal’, the location 1 cm distally was denoted
‘distal’, and both were marked with 4.0 vicryl. At the same level, the femoral
vein, femoral nerve and rectus femoris muscle segments were marked in the same
fashion. The clamping time was two hours. At the end of two hours, a
longitudinal incision was made on the anterolateral side of the leg and a 5-cm
segment of tibialis anterior muscle was excised. From the marked lines, the
femoral artery, vein, nerve and rectus femoris muscle were excised. The samples
were sent to the pathology laboratory for histological analyses. The animals
were euthanised after the procedure.
In
the tourniquet group, a ‘Blue 12 inch for child’ (reference no: 20-54-710, VBM
Medizintechnik GmbH, Sulz am Neckar, Germany) tourniquet was used. The standard
tourniquet time for all subjects was two hours and the pressure was 200 mmHg.8
The proximal and distal borders of the cuff were marked with a tissue pen.
After two hours, the tibialis anterior muscle sections were excised, as with
the clamp group. The tourniquet was released and removed, then the femoral
artery, vein, nerve and rectus femoris muscle were excised from the previously
marked cuff margins. In the control group, no clamp or tourniquet was used. A
longutidinal incision was made on the anterolateral side of the crus muscle. A
5 cm length of tibialis anterior muscle was excised. A longitudinal
anteromedial incision was made over the femoral neurovascular bundle. The
neurovascular bundle and rectus femoris muscle were marked with 4.0 vicryl
suture rope from the proximal and distal borders. Then the bundle and rectus
femoris muscle were excised. Histological examination of the common femoral
artery, vein, nerve, rectus femoris and tibialis anterior muscle was performed
(Figs 1, 2). The animals were euthanised. Areas with significant histological
findings were evaluated. In the clamp group, the arterial specimen was divided
into three groups: proximal, the clamp area in the middle, and distal. The aim
of this part of the study was to compare the normal tissue in the proximal part
of the ischaemic area, the damage to the arterial tissue where the clamp was
applied, and the changes due to ischaemia in the distal part of the clamp. In
the tourniquet group, the artery sample was divided into three similar pieces.
The aim of this part of the study was to evaluate the effect of pressure
differences on the tissue between the proximal and the middle part of the
tourniquet cuff, and to examine the effect of ischaemia on the distal side. In
both the tourniquet and the clamp groups, the vein, nerve and muscle tissue
were divided into three sections: proximal, middle and distal. In the clamp
group, the aim was to evaluate the tissue injury caused by dissection. In the
tourniquet group, the aim was to evaluate the effect of pressure difference
between the proximal and middle part of the tourniquet cuff, and to examine the
effect of ischaemia on the distal side. The tibialis anterior muscle tissue was
examined in all three groups to compare the ischaemic injury distal to the
extremity. Connective and adipose tissue were not evaluated because no
significant light microscopic findings were expected with short-term trauma and
hypoxia in these kinds of tissues. Six slides for the vessels and three slides
for the nerves and muscles were obtained per sample. Samples from each group were
fixed with 10% neutral-buffered formalin for one day and processed for standard
paraffin embedding. Serial sections (4-µm-thick slices) were
cut using a microtome. All of the sections were stained with haematoxylin and
eosin (H&E) and vessel sections with elastin van Gieson stain (Ventana) for
light microscopic examination (Nikon-Eclipse-80i-DS-Ri1). An automatic device
(Ventana, Benchmark XT) was used for histochemical staining. The photographs
were captured with a digital camera (Nikon-Eclipse-80i-DS-Ri1). The
pathologist, who was blinded to the slide numbers and groups, examined the
slides for tissue damage, including sections from the tibialis anterior and
rectus femoris muscles, the femoral nerve and the femoral artery and vein.
Degeneration
and inflammation were evaluated semi[1]quantitatively
for scoring skeletal muscle injury. Histological findings such as cytoplasmic
eosinophilia with loss of cytoplasmic structures, cytoplasmic vacuolation,
swelling, loss of striation, fragmantation and rupture were used for scoring
muscle degeneration. The score was: 1, findings are mild and focal; 2, moderate
and in some areas; 3, severe and common. The following criteria were used for
inflammation: score 0, no inflammation; 1, mild inflammation; 2, moderate
inflammation, and 3, severe inflammation. The scores were added and the total
muscle injury scores were calculated for each group. Degenerative changes in
the peripheral nerve fibres were determined semi-quantitatively according to
oedema and axonal degeneration using light microscopy. If the findings were
mild, the score was 1; moderate, 2; and severe, 3. Light microscopy gives
limited information without electron microscopy findings. Essentially we did
not expect serious damage to the nerve as the clamp or tourniquet was applied
for a short time only. Arterial injury was scored using the criteria in Table
1. Endothelial injury was scored using the same method applied for arteries and
venules. Venous intimal plaque was also evaluated. Using a quantitative
approach, intact endothelium in the 500-µm segment was
assessed by number of endothelial cells (NEC) in the arteries and venules.
Endothelial damage (score of endothelial ınjury: SEI) was assessed in the
venules and arteries. The continuity of the internal elastic lamina (score of
lamina elastica interna injury: SLEI) in the arteries was examined, as well as
the smooth muscle vacuolation in the medial layer (tunica media smooth muscle
vacuolation: TMSMV) in the arteries. Western blot analysis was used for protein
analysis. Tissue samples from the distal part of the clamp group (n = 7), the
distal part of the tourniquet group (n = 7) and the control group (n = 7) were
snap frozen in liquid nitrogen and stored at –80°C. Being a key regulator for
tissue injury TNF-α and also for loading control,
beta-actin antibodies were used for analysis and bands were determined using
the imaging system (Vilber Fusıon FX, France).
Statistical analysis
Descriptive
statistics were used to define continuous variables (mean, standard deviation,
minimum, median, maximum). Comparisons of independent variables with normal
distribution were performed using the Student’s t-test. Comparisons of two
independent and non-normal distributions were performed using the Mann–Whitney
U-test. The chi-squared test (or Fisher’s exact test at appropriate locations)
was used to examine the relationship between categorical variables. The
statistical significance level was determined as 0.05. The analysis was
performed using MedCalc Statistical Software version 12.7.7 (MedCalc Software
BVBA, Ostend, Belgium) and ordinary one-way ANOVA was performed using GraphPad
Prism version 7.0c (GraphPad Software, La Jolla California USA).
Results
For
the artery, the NEC values of the clamp and tourniquet groups were lower than
those of the control group (p ≤ 0.001, p = 0.007, respectively), while the
other parameters of the clamp and tourniquet groups were higher than those of
the control group (SEI, p ≤ 0.001 and p ≤ 0.001; SLEI, p ≤ 0.001 and p = 0.004;
TMSMV, p = 0.004 and p = 0.008; total score, p ≤ 0.001 and p ≤ 0.001,
respectively). When the clamp and tourniquet groups were compared, no
differences were found for all vascular parameters (Fig. 1C, D, Table 2). For
the vein, the NEC values of the clamp and tourniquet groups were lower than
those of the control group (both p ≤ 0.001). The SEI values were higher in the
clamp group than in the control group (p = 0.055). In the tourniquet group, all
values were higher than in the control group (p = 0.023) (SLEI, both p ≤
0.001). There was no difference between the distribution of plaque in the
distal, middle and proximal regions of vessels in the clamp and tourniquet
groups (Fisher’s exact test, p > 0.05) (p = 0.286, 0.265, 1.00,
respectively). No difference was found between the clamp and tourniquet groups
for all parameters. For nerve and muscle tissue, there were no differences
between the groups regarding femoral nerve injury scores, rectus femoris and
tibialis anterior degeneration, inflammation and total injury scores (p =
0.533, 0.876, 0.604, 0.756, respectively). There were also no differences
between the middle regions of vessels of the clamp group and the mean values of
the tourniquet group (Mann–Whitney U-test, p > 0.05). Protein levels were
evaluated by Western blotting. The samples were normalised using beta-actin
levels. The bands were analysed by densitometry and normalised using Image J
Software (National Institutes of Health, USA). Statistical analysis was carried
out by ordinary one-way ANOVA using GraphPad Prism version 7.0c (GraphPad
Software, La Jolla California USA) and no statistically significant differences
were found between the groups for TNF-α values (p = 0.1712)
(Fig. 3)
Discussion
To
our knowledge, this is the first study comparing tourniquet and arterial
clamping in the literature. We found no significant difference between tourniquet
and clamp methods regarding histological and inflammatory response in the
vessel. Therefore, the clamp method can be used in orthopedic oncological,
trauma and revision hip-joint surgeries that are unsuited to a tourniquet. The
external iliac artery or axillary artery can be clamped by a vascular surgeon
at the beginning of a revision joint surgery or resection of a proximal limb
tumour. With careful vessel dissection and the minimum pressure required for
occlusion, clamp-related complications can be avoided. Interrupting blood flow
using an arterial clamp or a tourniquet is associated with haemodynamic changes
and leads to inflammation, which triggers pathophysiological processes.10
Zammert et al. reported that with arterial clamp application, TNF-α played a crucial role in haemodynamic changes and was
associated with tissue injury.11 Although the behaviour of endotoxins after
clamping is unclear, Caty et al. showed that TNF-α was involved in the
initiation of injury.12 TNF-α has a central role
in initiating an inflammatory response by engaging multiple pathways,
especially mitogen[1]activated
protein (MAP) kinases and caspase proteases. MAP kinases increase TNF-α expression and induce a secondary response.13 Interactions
between MAP kinases and TNF-α contribute
significantly to tissue regulation for cell response in damage and cellular
homeostasis.14 MAP kinases, also known as stress-response kinases, are
triggered by environmental stressors. Mechanical damage of tissue activates the
MAP kinase (JNK and p38) pathway stimulated by TNF-α, and activated MAP kinases alter physiological responses in the
process of various diseases.15,16 Activation of JNK has been reported in
various pathological conditions such as heart failure and ischaemia– reperfusion injury. In a recent study, authors evaluated images
from histologically stained tissue sections obtained from rabbit and human
atria.17 In this study, interstitial fibrosis was evaluated by Masson’s
trichrome stain. Fibrosis was not expected in our study due to interruption of
blood supply for two hours, therefore immunohistochemical examination was not
used in our study. Our study indicated that increased TNF-α protein expression was associated with the tourniquet group;
although, when all groups were compared with each other, no significant
differences were found (Fig. 3). In this scope, our findings suggest that clamp
application is favourable to the use of a tourniquet. Longer tourniquet time
and higher inflation pressure were associated with higher complication risk.18
Also, higher age and co-morbidities, such as trauma, peripheral vascular
disease and hypertension, elevated the rate of complications.19 Debates
therefore continue about the safety limits associated with pressure and
duration. However, most authors suggest that 1.5 to two hours with 200–250 mmHg
inflation pressure is appropriate for healthy, normotensive patients.8 When
these safety limits are exceeded, complications may be encountered.2 Another
parameter that affects the pressure is the cuff width. The cuff should be as
wide as possible, and it should not encroach upon the surgical site.2 Contrary
to this general belief, a report suggests that muscle damage increases with
wide cuffs.20 There are several reports regarding nerve injury related to the
use of tourniquets.21,22 Nerve tissue is more sensitive to mechanical pressure
than muscle tissue, and two studies showed that injury was severe at the
proximal and distal edges because of shear stress.18,22 There was a strong
correlation between mechanical pressure duration and nerve injury. Even below
30 minutes of inflation time, paralysis has been reported. Also, after each
30-minute increase in duration, there was a three-fold increase in neurological
complications.22 Muscle tissue is more sensitive to prolonged ischaemia than
nerve tissue. Moreover, the injury is severe beneath the cuff.8 Animal studies
have shown that tourniquets are related to decreased muscle force beneath and
distal to the cuff and are directly proportional to cuff pressure.23 Contrary
to nerve and muscle complications, vascular complications due to tourniquet use
are rare. However, some reports suggest the opposite. For example, Rush et al.
found that direct pressure can cause fracture of plaque formation or thrombosis
in atherosclerotic vessels.24 DeLaurentis et al. suggested not to use
tourniquets if there is a femoropopliteal aneurysm, femoral–popliteal bypass or
calcification. They also concluded that ischaemic pressure necrosis is an
additional mechanism of injury.25 Another report recommends avoiding tourniquet
use with poor distal pulses, capillary return or calcified vessels near the
application field.26 However, the reasons for this suggestion (whether because
of tourniquet-caused fractures or distortion-traction during surgery) are
unclear.27 Although various types of skin-protection paddings have been
produced, skin injury can be encountered at rates of 0.04–0.1%.28 Tourniquet
application therefore has several disadvantages. Nerve and muscle injury are
common complications and can occur, even with short inflation times.
Complication rates increase when the applied pressure is not adjusted to
systemic blood pressure, extremity diameter and cuff width. Tourniquets also
require regular calibration and incorrect calibrations can cause serious
complications. Vessel complications may be less rare, but additional nerve,
muscle and skin complications should be noted. No international quantitative
unit can repeatedly be used in experimental studies to measure clamp
pressure.29 In an experimental study, to standardise the clamp pressure between
the subjects, the authors noted the lowest notch number at which the clamp did
not slip on the vessel but provided transient occlusion.9 In the same manner,
to standardise the pressure, we used the same micro-clamp, which is the
smallest available, to occlude the vessel for all subjects. There are also
experimental studies examining vessel damage due to clamp application.9 In an
experimental study, four DeBakey vascular clamps were applied to eight carotid
arteries of four adult sheep for durations of 15, 30, 45 and 60 minutes,
respectively. A significant and ongoing increase in endothelial damage was seen
at 15 minutes; the damage was maximal after 30 minutes. The authors concluded
that there are four variables to determine the force needed to occlude a
vessel: vessel diameter, blood pressure, vessel elasticity and blade contact
area. The severity of injury varies according to duration, pressure,
intraluminal flow pattern, plaques and vessel elasticity.9 There are some
limitations associated with our study. First, this study did not include a
group of participants that were allowed to live after the study (to investigate
the late histological changes for both the tourniquet and clamp application).
This group of animals could have provided more information about To
our knowledge, this is the first study comparing tourniquet and arterial
clamping in the literature. We found no significant difference between
tourniquet and clamp methods regarding histological and inflammatory response
in the vessel. Therefore, the clamp method can be used in orthopedic
oncological, trauma and revision hip-joint surgeries that are unsuited to a
tourniquet. The external iliac artery or axillary artery can be clamped by a
vascular surgeon at the beginning of a revision joint surgery or resection of a
proximal limb tumour. With careful vessel dissection and the minimum pressure
required for occlusion, clamp-related complications can be avoided.
Interrupting blood flow using an arterial clamp or a tourniquet is associated
with haemodynamic changes and leads to inflammation, which triggers
pathophysiological processes.10 Zammert et al. reported that with arterial
clamp application, TNF-α played a crucial role in haemodynamic
changes and was associated with tissue injury.11 Although the behaviour of
endotoxins after clamping is unclear, Caty et al. showed that TNF-α was involved in the initiation of injury.12 TNF-α has a central role in initiating an inflammatory response by
engaging multiple pathways, especially mitogen[1]activated protein (MAP)
kinases and caspase proteases. MAP kinases increase TNF-α expression and induce a secondary response.13 Interactions
between MAP kinases and TNF-α contribute
significantly to tissue regulation for cell response in damage and cellular
homeostasis.14 MAP kinases, also known as stress-response kinases, are
triggered by environmental stressors. Mechanical damage of tissue activates the
MAP kinase (JNK and p38) pathway stimulated by TNF-α, and activated MAP kinases alter physiological responses in the
process of various diseases.15,16 Activation of JNK has been reported in
various pathological conditions such as heart failure and ischaemia–
reperfusion injury. In a recent study, authors evaluated images from
histologically stained tissue sections obtained from rabbit and human atria.17
In this study, interstitial fibrosis was evaluated by Masson’s trichrome stain.
Fibrosis was not expected in our study due to interruption of blood supply for two
hours, therefore immunohistochemical examination was not used in our study. Our
study indicated that increased TNF-α protein expression
was associated with the tourniquet group; although, when all groups were
compared with each other, no significant differences were found (Fig. 3). In
this scope, our findings suggest that clamp application is favourable to the
use of a tourniquet. Longer tourniquet time and higher inflation pressure were
associated with higher complication risk.18 Also, higher age and co-morbidities,
such as trauma, peripheral vascular disease and hypertension, elevated the rate
of complications.19 Debates therefore continue about the safety limits
associated with pressure and duration. However, most authors suggest that 1.5
to two hours with 200–250 mmHg inflation pressure is appropriate for healthy,
normotensive patients.8 When these safety limits are exceeded, complications
may be encountered.2 Another parameter that affects the pressure is the cuff
width. The cuff should be as wide as possible, and it should not encroach upon
the surgical site.2 Contrary to this general belief, a report suggests that
muscle damage increases with wide cuffs.20 There are several reports regarding
nerve injury related to the use of tourniquets.21,22 Nerve tissue is more
sensitive to mechanical pressure than muscle tissue, and two studies showed
that injury was severe at the proximal and distal edges because of shear
stress.18,22 There was a strong correlation between mechanical pressure
duration and nerve injury. Even below 30 minutes of inflation time, paralysis
has been reported. Also, after each 30-minute increase in duration, there was a
three-fold increase in neurological complications.22 Muscle tissue is more
sensitive to prolonged ischaemia than nerve tissue. Moreover, the injury is
severe beneath the cuff.8 Animal studies have shown that tourniquets are
related to decreased muscle force beneath and distal to the cuff and are
directly proportional to cuff pressure.23 Contrary to nerve and muscle complications,
vascular complications due to tourniquet use are rare. However, some reports
suggest the opposite. For example, Rush et al. found that direct pressure can
cause fracture of plaque formation or thrombosis in atherosclerotic vessels.24
DeLaurentis et al. suggested not to use tourniquets if there is a
femoropopliteal aneurysm, femoral–popliteal bypass or calcification. They also
concluded that ischaemic pressure necrosis is an additional mechanism of
injury.25 Another report recommends avoiding tourniquet use with poor distal
pulses, capillary return or calcified vessels near the application field.26
However, the reasons for this suggestion (whether because of tourniquet-caused
fractures or distortion-traction during surgery) are unclear.27 Although various
types of skin-protection paddings have been produced, skin injury can be
encountered at rates of 0.04–0.1%.28 Tourniquet application therefore has
several disadvantages. Nerve and muscle injury are common complications and can
occur, even with short inflation times. Complication rates increase when the
applied pressure is not adjusted to systemic blood pressure, extremity diameter
and cuff width. Tourniquets also require regular calibration and incorrect
calibrations can cause serious complications. Vessel complications may be less
rare, but additional nerve, muscle and skin complications should be noted. No
international quantitative unit can repeatedly be used in experimental studies
to measure clamp pressure.29 In an experimental study, to standardise the clamp
pressure between the subjects, the authors noted the lowest notch number at
which the clamp did not slip on the vessel but provided transient occlusion.9
In the same manner, to standardise the pressure, we used the same micro-clamp,
which is the smallest available, to occlude the vessel for all subjects. There
are also experimental studies examining vessel damage due to clamp
application.9 In an experimental study, four DeBakey vascular clamps were
applied to eight carotid arteries of four adult sheep for durations of 15, 30,
45 and 60 minutes, respectively. A significant and ongoing increase in
endothelial damage was seen at 15 minutes; the damage was maximal after 30
minutes. The authors concluded that there are four variables to determine the
force needed to occlude a vessel: vessel diameter, blood pressure, vessel
elasticity and blade contact area. The severity of injury varies according to
duration, pressure, intraluminal flow pattern, plaques and vessel elasticity.9
There are some limitations associated with our study. First, this study did not
include a group of participants that were allowed to live after the study (to
investigate the late histological changes for both the tourniquet and clamp
application). This group of animals could have provided more information
about
Conclusion
This
study found no difference between the tourniquet and clamp methods regarding
vessel injury. The tourniquet is not ideal for the proximal field of
extremities. In addition, it might cause vessel complications in the presence
of underlying vascular disease. Also, skin, muscle and nerve complications
could be encountered. Complication rates increased when the applied pressure
was not adjusted to systemic blood pressure, extremity diameter and cuff width.
When using a clamp in clinical practice, iatrogenic vessel injuries may be
encountered during vascular dissection and may require peri-operative vascular
surgeon consultation. However, clamp-related complications can be avoided with
careful dissection and the minimum pressure required for occlusion. The arterial
clamp method can be safe and useful, without tourniquet-related complications,
for proximal extremities, where there is not enough space for a tourniquet.
Bezmialem Vakif
University Scientific Research Projects supported the data collection of this study,
project no: 2016/17. We thank Neslihan Gokmen for assistance with the
statistics used in this report
 لغة
لغة Türkçe
Türkçe English
English Arabic
Arabic Germany
Germany Russian
Russian