Bioactive Glass Graft vs Allograft in Benign Bone Lesions: A Retrospective Comparative Study
Introduction
For filling bone defects after curettage, autologous bone grafting offers osteoconductive, osteoinductive, and osteogenic properties. Döring et al [4] demonstrated that filling bone defects with autologous bone after curettage resulted in a superior recurrence-free survival compared to allogenous bone, cement, or no filling, supporting its use as the gold standard [6,17]. However, autografts are associated with donor-site morbidity, limited availability, and potential complications [1]. The search for suitable alternatives has led to the investigation of various bone graft substitutes, including bioactive glass (BG). Bioactive glass, composed of silica-based material, possesses several properties that make it an attractive option for bone regeneration. It exhibits osteoconductive and osteoinductive potential, promoting the formation of new bone [11]. Additionally, BG has demonstrated antimicrobial properties, which can be beneficial in cases of infected bone lesions or in preventing postoperative infections [18]. The slow resorption rate of BG may also offer an advantage in the treatment of benign bone cysts, which are prone to recurrence in growing children [9]. Studies have investigated the use of BG in various clinical settings, including the treatment of chronic osteomyelitis and bone defects, but its comparison to allograft in the context of benign bone lesions remains less explored [10,18]. Some studies have examined the use of BG in benign bone lesions yet lack a direct comparison to allograft, limiting conclusions about their efficacy [7,11]. In preclinical animal models, the biocompatibility of 45S5 BG has been shown to promote bone consolidation and integration [6,12,14,15], but further research is needed to establish its effectiveness against allograft in clinical settings. Therefore, there is a need for well-designed studies comparing outcomes of BG and allograft in the treatment of benign bone lesions. We sought to conduct a comparative analysis between allograft and BG as filling materials following intralesional curettage in the management of benign bone tumors. We hypothesized that 45S5 BG granules would exhibit comparable efficacy to allogeneic bone grafts in facilitating bone regeneration subsequent to tumor curettage procedures. We sought to examine (1) radiographic assessment of healing of the cysts treated with either allograft or BG using the Neer radiographic score and (2) assessment of functional outcomes following surgical treatment using the MSTS score.
Methods
After obtaining Institutional Review Board approval, we performed a retrospective review of electronic medical records of 81 patients who underwent curettage and bone grafting from 2018 to 2022. Inclusion criteria were: (1) preoperative diagnosis of a bone lesion amenable to curettage and grafting and (2) use of BG or allograft. Patients who underwent curettage without the use of BG or allograft or participated in another study that would interfere with the results of this study (eg, one that used bone autograft, synthetic bone grafts, or orthobiologic compounds) were excluded. A total of 42 patients met these criteria and were divided into 2 groups: the BG group (n = 21) and the allograft group (n = 21). Patients treated with allograft underwent reconstruction with fresh-frozen morselized femoral head allograft, while those in the BG group received 45S5 BG granules (Meta Bioengineering and R&D Services Inc., Istanbul, Turkey) as the bone defect filler following curettage. The femoral heads were obtained from patients undergoing primary hip arthroplasty for osteoarthritis at our institution. Our institutional bone bank procedures and protocols were followed, including consent from the patient for donation, perioperative prophylactic antibiotics, and surgery in an ultra-clean air environment. Sterility was appraised and maintained through microbiological swabs and bone samples taken from the femoral head and neck, respectively. The femoral head was discarded if any microbiological tests returned positive. Surgical intervention was primarily indicated in cases involving pain, limited range of motion, risk of fracture, or lesion situated near the joint line. All surgical procedures were conducted by a single experienced orthopedic oncology surgeon. A cortical window was created using an osteotome, facilitating access to the lesion. The tumor mass was meticulously curetted and subsequently submitted for histopathological examination. The cyst’s contents were systematically evacuated using curets, and the lining membrane was ablated with a high-speed burr. A 5% phenol solution was applied to the cyst cavity via cotton swabs and neutralized with saline irrigation. The resultant cavity was filled with either the morselized allograft or the BG. Intraoperative fluoroscopy was employed to verify the complete filling of the cyst cavity. In cases involving a heightened risk of pathological fracture, locking plate osteosynthesis was implemented to augment stability. The mean volume of implanted BG was 14.81 ± 11.06 cc. The mean volume of implanted allograft was 44.05 ± 50.22 cc. All patients underwent preoperative assessment (baseline) and postoperative evaluations at 6 weeks and 3, 6, 12, and 24 months. Follow-up visits incorporated clinical examinations and radiographic imaging (Figs. 1 and 2). Limb function was assessed using the Musculoskeletal Tumor Society (MSTS) score for the upper or lower extremity at the final follow-up [5]. Preoperative magnetic resonance imaging (MRI) was performed in all cases. The primary outcome for this study was the radiologic assessment of healing of the treated cysts. The modified Neer radiographic classification system was employed to evaluate bone healing outcomes, stratifying them into 4 categories: healed, healed with a defect, persistent cyst, and recurrent cyst [3,8,21]. A healed outcome was defined as a cyst demonstrating complete osseous infilling, potentially accompanied by a minor radiolucent area measuring less than 1 cm. In cases classified as healed with a defect, static radiolucent zones were observed, constituting less than 50% of the bone diameter, with adequate cortical thickness to mitigate fracture risk. A persistent cyst was characterized by a radiolucent area exceeding 50% of the bone diameter, coupled with a thin cortical rim and no discernible increase in cyst dimensions, necessitating ongoing activity restriction or potential reintervention. A recurrent cyst was defined as either the reappearance of a cyst in a previously obliterated region or the enlargement of a residual radiolucent area. All patients underwent radiographic assessment at the mentioned time intervals, including a 24-month radiograph.
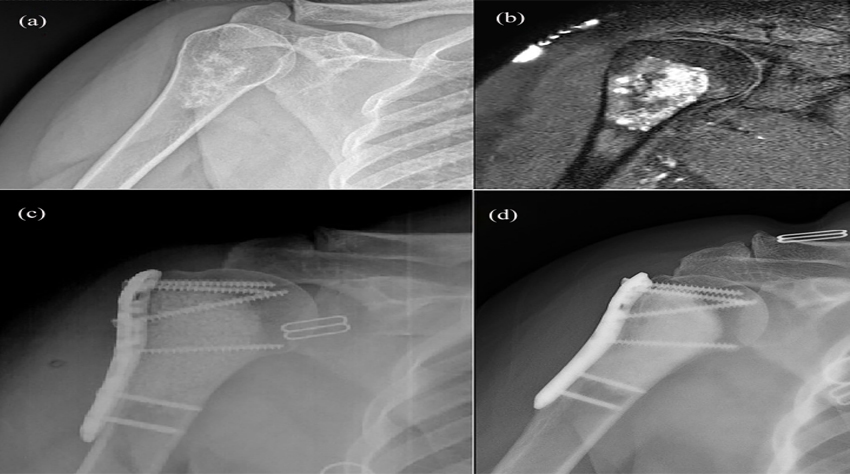
Fig. 1. An enchondroma in a 50-year-old woman. (a) Preoperative radiograph. (b) Preoperative T2-weighted MRI. (c) Radiograph
after intralesional curettage and filling of the defect using bioactive glass graft and locking plate osteosynthesis. (d) A radiograph taken
3 years after the procedure demonstrates successful healing without signs of recurrence.
The same surgeon who performed the surgeries and who
has expertise in musculoskeletal oncology performed radiographic assessments and documented all radiological and
functional examination findings.
The preoperative tumor volume was assessed utilizing
preoperative imaging studies, adhering to the methodological framework established by Prosser et al [14]. Computed
tomography (CT) scans and MRIs were employed to acquire
measurements of tumor diameter, volume, and distance
from the articular surface. Tumor volume was calculated
using the following spherical approximation: 0.52 × length
× breadth × height.
Functional outcome was assessed using the MSTS score,
initially introduced by Enneking et al [5]. This scoring system ranges from 0 to 30, with 0 indicating a complete loss
of function and 30 representing 100% function. The study
evaluated the MSTS score at the final follow-up visit (mean
2.76 years for the BG group and 3.95 years for the allograft
group), as assessed by the surgeon in the outpatient clinic,
and documented any complications, such as recurrence,
deep surgical site infection, or pathologic fracture.
Additionally, all patients answered questions about their
postoperative clinical course, their daily activity prior to
surgery, and when they resumed activities postoperatively.
Fig. 2. An enchondroma in a 50-year-old woman. (a) Preoperative radiograph. (b) Preoperative T2-weighted MRI. (c) Radiograph
after intralesional curettage and filling of the defect using allograft bone. (d) A radiograph taken 6 years after the procedure
demonstrates successful healing without signs of recurrence.
Statistical Analysis
The normality of continuous variables was assessed using the Shapiro-Wilk test. As the variables did not show a normal distribution, the Mann-Whitney U test was used for inter-group comparisons. The Wilcoxon signed-ranks test was employed to compare preoperative and postoperative MTST scores. For comparing the change in preoperative and postoperative scores across groups, a repeated measures analysis of variance (ANOVA) model with time– group interaction was constructed. Categorical data analysis utilized the χ2 test and Fisher’s exact test. Data analysis was performed using TIBCO Statistica software, with a statistical significance level of 0.05. In order to detect a difference between the BG and allograft groups in preoperative and postoperative MSTS scores with an effect level of 0.25 (medium), 80% power, and 5% type I error, a total of 34 patients were included in the study. A total of 21 patients were included in each group, with an allocation ratio of 1:1, in order to allow for the possibility of 20% missing data. The requisite calculation was performed using the G*Power 3.1.9.7 software program. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines and checklist were used in reporting the data.
Results
The distribution of sexes was unequal, with a higher proportion of females in the BG group (15 females, 6 males)
than in the allograft group (10 females, 11 males). The most
common skeletal sites were the femur (9 in the BG the
allograft group) and humerus (7 in each group). The most
common types of cysts were unicameral bone cysts (5 in the
BG group, 7 in the allograft group) and enchondromas (12
in the BG group, 4 in the allograft group) (Table 1).
No significant difference in radiographic healing was
found between the BG and allograft groups in terms of postoperative Neer scores (P = .899; Table 2).
There was no difference in the MSTS scores between the
2 groups at the final follow-up. The mean preoperative
MSTS scores were 19.9 ± 5.8 and 19.05 ± 7.02 in the BG
and allograft groups, respectively (P = .632). The postoperative MSTS scores were 27.76 ± 3.06 and 27.33 ± 3.38
in the BG and allograft groups, respectively (P = .526). A
statistically significant improvement in MSTS scores was observed over time for both groups (P < .001), with no significant interaction between time and group (P = .829). Additionally, the mean improvement in MSTS scores for both groups did not reach the minimal clinically important difference (MCID) of 10 points, suggesting that the observed changes may not be clinically significant. The time to return to the previous activity level was similar between the BG group (17.95 ± 11.4 weeks) and the allograft group (18.95 ± 14.2 weeks; P = .899; Table 2). The complication rates were also comparable between groups (P = 1.00; Table 3).
Discussion
In comparing the healing of benign bone cysts treated with BG vs allograft cancellous bone, we found no differences in either the radiographic evidence of healing or the MSTS scores and a similar incidence of complications between the 2 groups. This suggests that BG may be a suitable alternative to allograft for the treatment of benign bone lesions.
Our study has several limitations. The retrospective design introduces the possibility of selection bias and limits control over confounding factors. The inclusion of various benign bone lesion types with differing recurrence rates contributes to sample heterogeneity, potentially influencing results. Although we performed subgroup analyses and adjusted for lesion type in our statistical models, the relatively small sample size may have limited the power to detect subtle differences between groups. Furthermore, the lack of anonymizing in the assessment of outcomes and the potential for detection bias in the evaluation of imaging studies should be considered when interpreting the results. The heterogeneity of bone lesion locations in this study, while potentially enhancing generalizability, may also introduce confounding factors that influence the comparison between BG and allograft, limiting the direct comparison between groups due to varying recurrence rates and other clinical factors. The MSTS score, while widely used, may not be the most appropriate measure for assessing functional outcomes across diverse anatomical locations. Finally, the 2-year follow-up period, while sufficient to assess short-term outcomes, may not capture long-term complications or recurrence patterns.
Our findings are consistent with previous studies suggesting comparable outcomes between BG and other bone graft substitutes in various clinical settings (Table 4). Previous research has suggested that BG may be useful in promoting bone healing and regeneration in both adult and pediatric populations [10,18,19]. The osteoconductive, osteoinductive, and angiogenic properties of BG may contribute to favorable outcomes in bone defect filling and fracture healing [10,18]. Moreover, the potential antimicrobial properties of BG offer another potential advantage in preventing postoperative infections and managing contaminated bone defects [2,18].
While we found no significant differences in MSTS scores between groups, suggesting that both BG and allograft bone may facilitate functional recovery after curettage of bone lesions, it is important to note that the observed changes in MSTS scores in both groups did not reach the MCID. This indicates that the clinical significance of these changes may be limited, possibly due to small functional deficits or the limitations of the MSTS score in assessing
functional outcomes across diverse anatomical locations. This is in line with the findings of Syvanen et al [20], who reported similar MSTS scores at 2-year follow-up in children treated with BG or allograft. Additionally, the comparable complication rates between the 2 groups suggest that BG may be a safe and well-tolerated bone graft substitute, consistent with previous reports [10,18].
While we found comparable clinical and radiographic outcomes for BG and allograft, their differing mechanisms of action should be considered. Allograft, being natural bone, possesses osteoinductive and osteogenic properties that contribute to cortical reconstitution—the restoration of the bone’s outer layer critical for structural integrity [1,7]. Bioactive glass is primarily osteoconductive, providing a scaffold for the ingrowth of new bone [1,13]. Future research incorporating quantitative measures of cortical reconstitution would enhance our understanding of the long-term remodeling patterns associated with these graft materials.
We found comparable time to return to previous activity levels between the BG and allograft groups, with no statistically significant difference observed. This finding suggests that both materials may allow for a similar functional recovery and rehabilitation process, enabling patients to resume their daily activities at a comparable pace. Our results are consistent with previous studies that reported no significant differences in the time to return to sports or work between patients treated with BG and with autograft or other bone substitutes [10,11,18]. This further suggests that BG, despite being a synthetic material, does not hinder functional recovery and allows for a timely return to pre-injury activity levels, similar to traditional bone grafting materials.
In conclusion, our retrospective study found comparable outcomes with BG and allograft for the treatment of benign bone lesions. Future research should focus on long-term follow-up and comparative studies with larger sample sizes to further elucidate the role of BG in the management of these lesions.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Human/Animal Rights
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration.
Informed Consent
Informed consent was waived from all patients included in this study by the institutional review board of Bezmialem Vakıf University (ID: 27092024-7-9).
Level of Evidence
Level III: Retrospective Therapeutic Study
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article as supplemental material.
ORCID iD
Mustafa Alper Incesoy https://orcid.org/0000-0002-0275-8101
 Dil
Dil Türkçe
Türkçe English
English Arabic
Arabic Germany
Germany Russian
Russian