Foot and Ankle Osteoid Osteomas
Foot and Ankle Osteoid Osteomas
Volkan
Gurkan, MD 1 , Ozgur Erdogan, MD
1Assistant Professor, Bezmialem
Faculty of Medicine, Istanbul, Turkey
2Orthopedist, Haydarpasa Numune
Education and Research Hospital, Istanbul, Turkey
ABSTRACT
Foot
and ankle osteoid osteomas (OOs) are often cancellous or subperiosteal and
rarely present with a periosteal reaction. Additionally, the large number of
disorders included in the differential diagnosis and the nonspecific findings
on radiographs complicate the diagnosis. We performed a manual search of the
senior surgeon’s hospitals’ operating room records for the terms “benign bone
tumor,” “foot,” “ankle,” and “osteoid osteoma” from January 2003 until December
2014. Of 87 surgically treated patients with lower extremity OOs, 9 patients
(11%) with foot or ankle OOs were included. The mean age at presen[1]tation
was 21 (range 6 to 30) years; all 9 (11%) patients were male. The patients were
evaluated for swelling, pain, trauma history, night pain, response to pain
relievers, duration of complaints, and interval to di[1]agnosis. The mean follow-up
period was 48 ± 24 months, and no recurrences had developed. The mean American
Orthopaedic Foot and Ankle Society scale score was 59.04 ± 11 before surgery
and 91.56 ± 6 after surgery. The difference was statistically significant at p
≤ .0003. Most previous studies have been limited to case reports. The need for
findings from a case series was an essential determinant of our de[1]cision
to report our results. Patients usually have been treated conservatively, often
for a long period. However, delays in treatment cause social, economic, and
psychological damage. In conclusion, the pres[1]ence of atypical findings
on radiographs has resulted in a preference for magnetic resonance imaging
instead of computed tomography; however, the diffuse soft tissue edema observed
on MRI can lead to the use of long-term immobilization and a delay in the
diagnosis. © 2017 by the American College of Foot and Ankle Surgeons. All
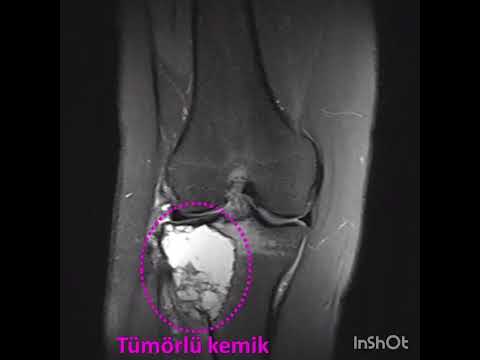
rights reserved. Osteoid osteoma (OO) is a vascularized, osteogenic, benign
bone tumor that was first defined by Heine in 1927 (1) and first described by
Jaffe in 1935 (2). OOs constitute 10% of all benign bone tumors and 19.4% of
all benign bone tumors in the foot and ankle, with a partic[1]ular
predilection for the talus and calcaneus (3,4). OOs can be divided into 3 types
according to their location: intracortical, cancellous, and subperiosteal (5).
Although long bone OOs cause an aggressive sub[1]periosteal reaction owing
to their intracortical location, foot OOs often occur in the cancellous bone or
subperiosteally and might not cause a periosteal reaction (5,6). Because of
these subtle radiologic find[1]ings,
the complex anatomy of the ankle and foot with the wide array of disorders
included in the differential diagnosis, and the rarity of OOs in this region, a
delay can occur in diagnosing foot and ankle OOs. Thus, when a patient presents
with foot or ankle pain that is espe[1]cially
longstanding, cannot be diagnosed, and is resistant to medical treatment, the
presence of an OO should be considered (7). In the present study, we
retrospectively evaluated the epidemiology, radio[1]logic features, surgical
treatment options (including open and percutaneous methods), and functional
outcomes of foot and ankle OOs. Most previous studies were limited to case
reports; the largest study (8) was a review reported in 2015 and was also based
substan[1]tially
on case reports. The need for the findings from a case series was an essential
determinant of our decision to report our series.
Patients and Methods
The
study was performed in accordance with the ethical standards of the Declaration
of Helsinki. All patients provided informed consent before inclusion in the
study, and a local ethics committee approved the study protocol. The present
retrospective study found 87 surgical[1]ly
treated patients with a preoperative diagnosis of a lower extremity OO from
January 2003 to December 2014. We performed a manual search of the senior
surgeon’s (V.G.) operating records for the terms “benign bone tumor,” “foot,”
“ankle,” and “osteoid osteoma.” Of the 87 patients, 9 patients (11%) had a foot
or ankle OO and were included in the present study. The patient data reviewed
included sex, age, site of the lesion, clinical and radiologic findings,
swelling, pain, response to pain relievers, duration of complaints, interval to
diagnosis, biopsy and
treatment
modality, and functional results. The preoperative and post[1]operative
clinical outcome scores were calculated using the American Orthopaedic Foot and
Ankle Society (AOFAS) scale score (9). Patients who had undergone previous
percutaneous or open surgical treat[1]ment
with recurrence were excluded from the study. Preoperative radiographs,
computed tomography (CT), magnetic resonance imaging (MRI), and scintigraphy
examinations were performed
Statistical Analysis
Statistical analysis was performed using
SPSS software (IBM, Armonk, NY) using an unpaired Student’s t test and the
Fisher exact test. Statistical significance level was set at p ≤ .05
Results
The
mean age was 21 (range 6 to 30) years, and all the patients were male (Table).
Statistical significance was not found for age, similar to the finding for our
lower extremity long bone OO patients (p ≤ .33). The lesion locations were as
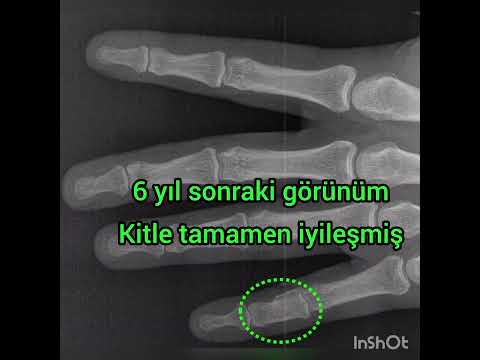
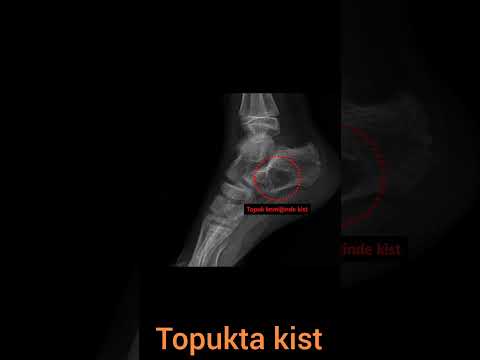
follows: calcaneus in 4 (44%), talus in 2 (22%), distal fibula in 1 (11%),
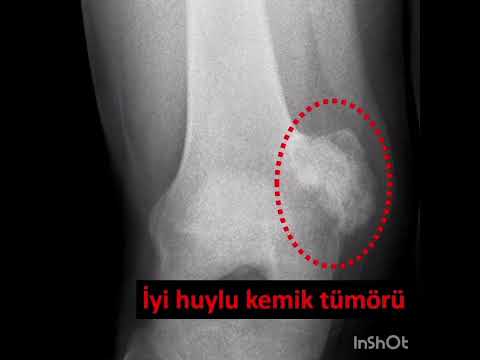
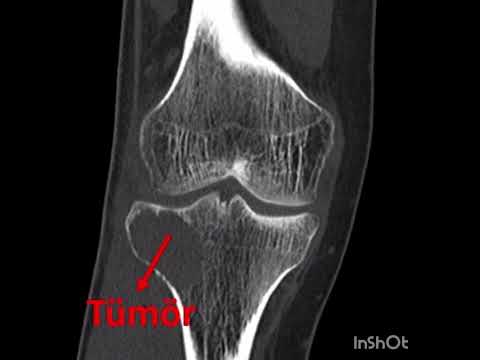
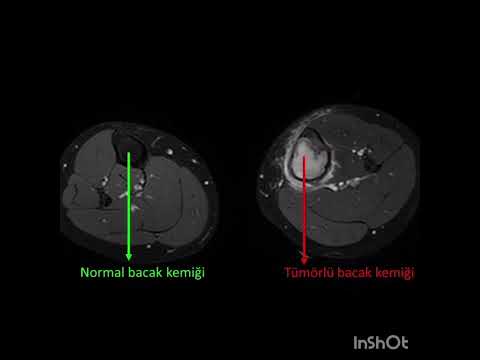
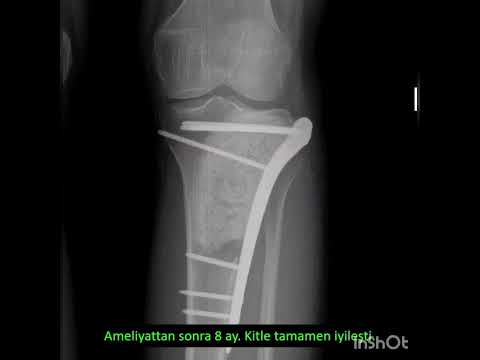
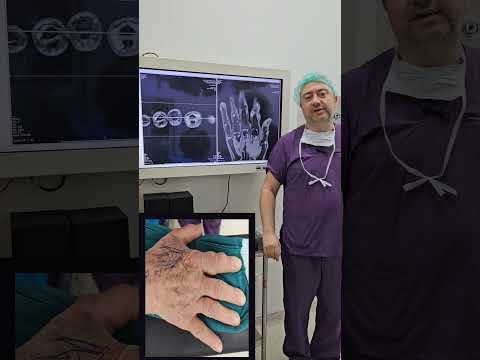
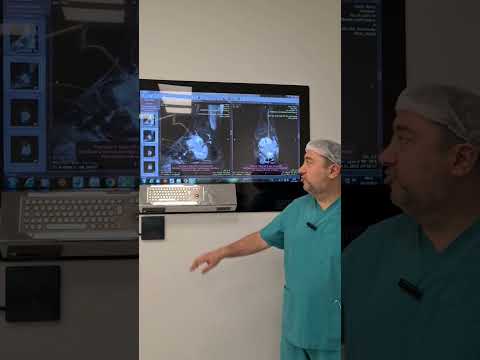
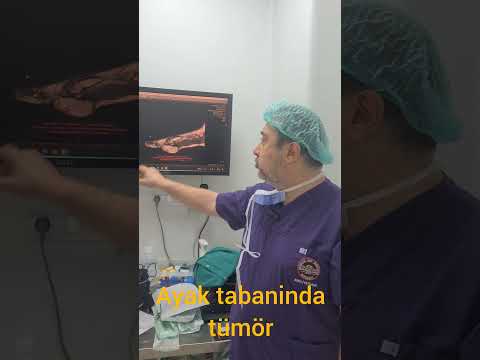
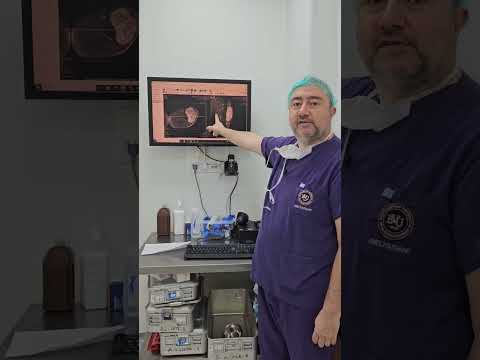
metatarsal in 1 (11%), and cuboid in 1 (11%; Figs. 1–3). The mean interval to
the diagnosis was 18 (range 12 to 48) months. All patients reported night pain,
localized tenderness, a response to pain relievers, pain with weightbearing,
local swelling, and an antalgic gait. Slight erythematous changes and a local
skin temperature in[1]crease
were present in 2 patients (22%). The complete blood count,
erythrocyte
sedimentation rate, and C-reactive protein levels were normal in all 9
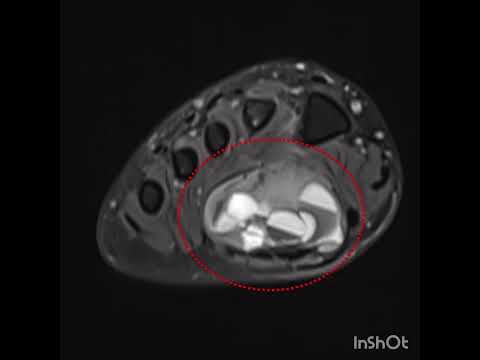
patients. All patients had undergone plain x-ray films and CT, 4 (44%) had undergone
MRI, and 4 (44%) had undergone scin[1]tigraphy
using single photon emission CT (SPECT)-CT. Treatment included the following:
en bloc resection in 3 (33%), unroofing and curettage with “burr down” in 2
(22%), curettage with cortical peeling in 2 (22%), and radiofrequency ablation
(RFA) in 2 (22%). After the team, which included an orthopedic oncology
surgeon, a musculoskeletal interventional radiologist, and an anesthesiologist,
was formed at the senior author’s (V.G.) institute to perform RFA, 2 patients
(22%) with a foot or ankle OO underwent RFA. Another 2 patients (22%), who had
been considered for RFA, were found not to be suitable because of the anatomic
proximity of the nidus to the neurovascular tissues or ar[1]ticular
surfaces. RFA was performed by consultant musculoskeletal radiologists. Before
ablation, a CT-guided needle biopsy of the lesion was performed for pathologic
diagnosis. The location of the lesions was as follows: subcortically cancellous
in 6 (66%), cancellous in 2 (22%), and cortical in 1 (11%). Of the 9 lesions, 8
(89%) were extraarticular and 1 (11%) was intraarticular. The mean nidus
diameter was mea[1]sured
using the CT images and was 6.8 (range 5 to 11) mm. Eight patients (89%)
reported pain relief after the procedure. One patient (11%) experienced
persistent pain for 6 months after surgery despite open curettage. SPECT-CT was
repeated and showed a residual nidus with increased uptake. This patient
underwent CT-assisted RFA 6 months after the initial procedure. Two patients
(22%) who had un[1]dergone
open surgery developed temporary superficial wound problems, which healed
without any surgical intervention. At the final follow-up examination, at a
mean of 48 ± 24 months after the initial procedures, no recurrences had
developed. The mean AOFAS scale score was 59.04 ± 11 before surgery and 91.56 ±
6 after surgery, and the dif[1]ference
was statistically significant (p ≤ .0003; Table).
Discussion
Few
studies of OOs have been reported, and the largest was a sys[1]tematic
review (8) reported in 2015, which was also based substantially on case reports
(64 of the 94 included studies were case reports). To the best of our
knowledge, most case series were also limited to small numbers of patients.
Zouari et al (10) reported on 7 patients (5.2%) with a foot or ankle OO of 133
patients with OOs, and Rehnitz et al (11) reported on 3 patients (4%) with a
foot or ankle OO of 72 pa[1]tients
with OOs. Therefore, a large number of prospective randomized trials are still
needed to determine the best evidence-based medicine. OOs are usually seen in
patients aged <40 years, and most pa[1]tients
will be <25 years, with males predominating at a ratio of 3:1. The male
predominance is valid for foot and ankle OOs; however, to the best of our
knowledge, no studies regarding the male predomi[1]nance have been reported.
Our results are consistent with the published data regarding the male
predominance and mean age. The most common symptom in OO patients was pain that
increased in sever[1]ity
at night and that responded well to prostaglandin inhibition; swelling was the
second most common symptom (12,13). It has been thought that the swelling is
related to the rich vascular supply of the tumor or the increased soft tissue
and vascular permeability that results from the presence of prostaglandins in
the mass (12,14). In 2 pa[1]tients
with redness of the skin, the lesion was located close to the skin
and
scintigraphy revealed intense activity. It has been thought that the pressure
and edema resulting from the lesion cause the pain by stimulating the
surrounding nerve fibers. In an immunohistochemi[1]cal study by O’Connell et
al (15), more nerve fibers than were expected were found surrounding the nidus
and reactive zone. Consistent with previous studies, all our patients
experienced night pain, and in 6 pa[1]tients
(66%), this pain was relieved by nonsteroidal antiinflammatory drugs. This
finding is consistent with the review by Jordan et al (8). The reported
incidence of OO has ranged from 2% to 10% in the foot (16), followed by the
calcaneus (2.7%), phalanx (2%), and metatar[1]sals (1.7%). In contrast to
the results reported by Jackson et al (17), we found that the most frequently
involved bone was the calcaneus (n = 4; 44%). OO has 3 histologic types:
cortical, cancellous, and sub[1]periosteal
(5). OOs tend to occur intracortically in the long bones and cause an excess
subperiosteal reaction. In contrast, they mostly develop in cancellous or
subperiosteal locations in the foot, where they cause a minimal periosteal
reaction (6). Cancellous OOs were present in 8 of our patients (88%), in
agreement with the findings from other studies, and 6 patients (75%) had
subcortical OOs. Houdek et al (18) classi[1]fied the lesions of their
patients as intracortical, periosteal, or subcortical (endosteal) according to
the relationship of the nidus to the cortex, instead of whether it was
subperiosteal or cancellous. Three of their patients’ lesions (27%) were intracortical
and 8 (73%) were sub[1]cortical
and were classified as the subcortical type of cortical lesion (18). This is
consistent with our results. However, controversy remains regarding whether the
localization should be described as cortical or cancellous. Although the
clinical presentation is often typical and di[1]agnostic, in some cases,
the nidus formation will not be seen on plain radiographs. This could have
resulted from transposition of the small bones (anatomic complexity of the
foot), the lack of a periosteal re[1]action,
cortical thickening, a longer time required for nidus formation in the foot and
ankle, a lower periosteal response against lesions ex[1]tending into the joint, and
the transposition of OOs that have settled close to the joint with synovial
tissue (16,19). Thus, the need to rule out many diseases, including ankle
distortions, monoarticular arthri[1]tis,
anterior impingement syndrome, tarsal spur, osteomyelitis, stress fracture,
eosinophilic granuloma, and sarcoma, has led to the prefer[1]ence
for using MRI instead of CT. Also, peroneal spasm and foot extensor
tenosynovitis have been added to the differential diagnosis (20). However, the
diffuse soft tissue edema observed on MRI can lead to the use of long-term
immobilization and a delay in the diagnosis (8). The mean interval to the
diagnosis was 18 (range 12 to 48) months in our series, similar to that
reported by Jordan et al (8) in their sys[1]tematic review. Also, the
mean delay between the initial presentation and the diagnosis was 22 (range 1 to
120) months. The patients had been treated conservatively for long periods, and
the delays had caused social, economic, and psychological damage (21). For
patients pre[1]senting
with the typical night pain responsive to pain relievers, who are in the high-risk
age group, and in the absence of suggestive find[1]ings of OO on radiographs,
thin-slice CT should be performed, in addition to MRI, for advanced imaging
studies. The bone marrow edema signal commonly seen with OO, which can be
intensely visualized using MRI, can mask the typical bony features of the
lesion, which are nearly pathognomonic on CT. Also, the diagnosis of OO can be
challenging using MRI alone. Failure to diagnose an OO using MRI occurred in 3
of our patients (33%), similar to previous reports (4,22). Therefore, MR
appears
to lack the specificity for diagnosing OO in a significant pro[1]portion
of patients. Farid et al (23) concluded that compared with bone scanning alone
the use of SPECT images with a low-dose CT tech[1]nique improved anatomic
localization and provided more precise morphologic information. A recent study
comparing SPECT images with low-dose CT and bone scans to diagnose OOs at all
body sites re[1]ported
that SPECT had greater sensitivity and specificity (both 100%) compared with CT
(sensitivity 77.8%; specificity 92.3%) and bone scans (sensitivity 100%;
specificity 38.4%) (24). Similarly, in 1 patient, we could not define the
lesion although plain radiographs, CT, MRI, and bone scanning were performed.
However, SPECT-CT used together with bone scanning, identified the lesion as an
OO. Even using advanced imaging studies, a diagnosis will not be made for 11%
of the OO lesions (25). These suspected OOs that could be not diagnosed
radiologi[1]cally
can be accurately diagnosed using SPECT-CT; however, further investigation is
required. The pathophysiology of OOs remains par[1]tially unclear. Atypical
cellular and trabecular components of the OO nidus can resemble neoplasia,
because they are small and have self[1]limiting
characteristics that resemble the inflammatory process (26). Vancamp et al (27)
postulated that the nidus is a reactive lesion that occurs in response to
trauma or is an unusual healing and vascular[1]ization process. more
studies are needed regarding the relationship between OO and trauma; however, a
history of trauma was present in one third of all cases (11). Also, the
similarity to the inflammatory process suggests an association with a history
of trauma (22,27). Ad[1]ditionally,
pain related to an OO has developed 2 to 8 years after trauma. Kayser et al
(28) hypothesized that many OOs will originate in a sub[1]periosteal location and
later appear as intracortical or medullary lesions. They termed this inward
migration a “shift of nidus.” They also ex[1]plained that this migration
involves bone, which continues with subperiosteal deposition and endosteal
erosion (28). We believe this argument also supports the trauma hypothesis. In
our series, a history of trauma was present in 4 patients (44%), and the mean
time between the trauma and pain presentation was 44 (range 30 to 52) months.
OO is generally seen in patients aged <40 years. This trend could also be
relevant for bone remodeling. It is known that the balance is in favor of bone
formation until the third decade and that bone destruction becomes dominant in
the fifth decade and beyond and the bone mass decreases. The patients in our
series had a mean age of 21 (range 6 to 30) years, similar to that reported in
other studies. The nidus con[1]tains
woven bone and a highly vascular stroma of connective tissue centrally, with
dilated capillaries. The formation of an OO nidus and the process of
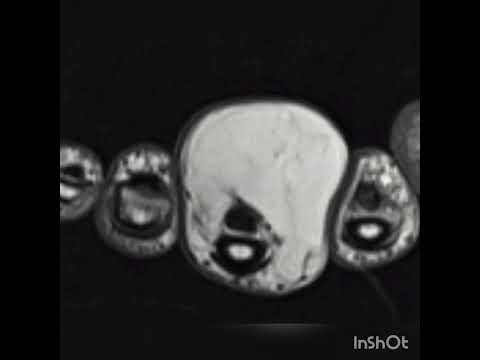
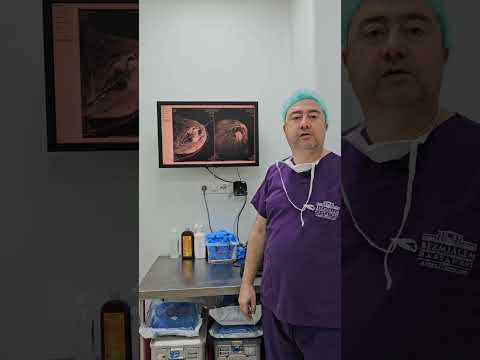
intramembranous (IM) healing are histologically similar (Figs. 4 to 6). IM
ossification occurs during flat bone formation and during the healing process
of a fracture treated with open reduction and inter[1]nal fixation. Adil et al
(29) hypothesized that invagination of the periosteum during fractures,
reduction, or pinning might be a pre[1]disposing
factor for the development of OO. Thus, the nidus of an OO might be an atopic
IM ossification area in the bone. It is unknown why the OO nidus does not
become mature. We suggest that the problem is related to either matrix
mineralization (transformation of amor[1]phous
calcium into hydroxyapatite) or collagen type 1 synthesis and organization.
Biochemical and histologic investigations of the nidus using electron
microscopy techniques are required to reveal the
histologic
differences in the collagen array and mineralization between the OO nidus and
the fibrous dysplasia, hyperthyroid, and fracture callus. Prostaglandin E2 is a
bone-resorbing cytokine secreted by os[1]teoblasts
and causes the typical pain associated with an OO. Thus, an imbalance in
prostaglandin E2 secretion or structure could be present that prevents nidus
maturation. We also suggest that insulin-like growth factor 1, which stimulates
collagen synthesis, and transform[1]ing
growth factor-β, which promotes osteoid matrix
synthesis, should be further investigated in studies of the pathophysiology of
OOs. Per[1]cutaneous
thermal destruction of the vascular-rich nidus is the current treatment of
choice and can be performed using a laser or RFA, with a success rate of
>90% (30). However, in selected cases, the proxim[1]ity to the chondral surface
or neurovascular structures should change the preference to an open technique.
The recurrence rates with both open and percutaneous techniques have ranged
from 0% to 15%, with similarly successful results. However, with the open
technique, the return to work and full weightbearing will require weeks, the
risk of fracture is greater, and the severity and incidence of postoperative
pain are greater (31). One patient (11%) in our series experienced persis[1]tent
pain for 6 months after surgery, despite open curettage. He underwent
CT-assisted RFA because of a residual nidus. After RFA, the patient was
asymptomatic. The success of this procedure depends on an accurate
preprocedural diagnosis and the precise anatomic local[1]ization with CT. This
patient is an example of the importance of CT[1]guided techniques. In our
study, 2 patients (22%) were treated with CT-guided RFA. None of these patients
developed recurrence or ex[1]perienced
persistent pain, and the mean AOFAS scale score improved from 58 (range 56 to
60) to 94 (range 92 to 96). The advantages of percutaneous techniques include
controlled ablation of the tumor nidus with minimal damage to the adjacent bone
tissue and performance as an outpatient procedure, which also allows for
immediate weightbearing and return to daily living. In addition, when the
average costs of hospitalization and treatment of OO using RFA and surgical
excision were compared, RFA was less expensive. RFA-related com[1]plications
include skin burns, nerve damage, reflex sympathetic dystrophy, cellulitis, and
thrombophlebitis. Thus, RFA should not be used for lesions near a neurovascular
bundle (<1.5 cm distance) (31). No complications or recurrence had developed
within a mean follow[1]up
period of 36 months in our RFA group; however, late recurrence is possible with
a longer follow-up duration. The retrospective study design and small number of
cases could be considered a weakness of our study; however, the rarity of foot
and ankle OOs makes it difficult to plan a prospective study with a large
number of cases. However, wide prospective randomized trials are still required
to determine the best evidence-based medicine. In conclusion, difficulties can
be experienced in the diagnosis of OO. The underlying reasons include the large
number disorders in the differential diagnosis and the nonspecific findings of
periarticu[1]lar
lesions on radiography. MRI has been preferred to CT to determine the cause of
foot and ankle pain when performing additional imaging studies. However, the
pathognomonic bone findings in OOs that can be visualized using CT will be
concealed by the peripheral edema seen on MRI. Thus, for patients presenting
with the typical night pain that is responsive to pain relievers and who are in
the age group at risk, even in the absence of suggestive findings for OOs on
radiographs, advanced imaging studies should include thin-slice CT, in addition
to MRI
 Sprache
Sprache Türkçe
Türkçe English
English Arabic
Arabic Germany
Germany Russian
Russian